Cleaning Systems for Filter/Dryers
.jpg?width=315&height=421&name=External_spray_ring%20(1).jpg) Clean-in-place (CIP) is a design principle commonly used in industries that require frequent internal cleaning of their process equipment and depend on a high level of cleanability to maintain their systems in order to meet strict hygienic standards. This includes but is not limited to pharmaceuticals, fine chemicals, processed food, beverage and cosmetics.
Clean-in-place (CIP) is a design principle commonly used in industries that require frequent internal cleaning of their process equipment and depend on a high level of cleanability to maintain their systems in order to meet strict hygienic standards. This includes but is not limited to pharmaceuticals, fine chemicals, processed food, beverage and cosmetics.
Although there aren’t strict guidelines to differentiate the terms from one another, the three main categories for cleaning in-place are listed here by their level of cleanliness:
- Wash-in-Place – WIP generally refers to equipment receiving full wetted coverage but may require some manual intervention along with the automated cleaning to guarantee total cleanability.
- Clean-in-Place – CIP denotes the cleaning of all interior surfaces including vessels, pipes, associated fittings, and other process equipment all without disassembly.
- Sterilization-in-Place – SIP requires the highest level of sanitation, in which all microorganisms and bacteria are removed and the vessel is considered totally disinfected. This incorporates heat into the cleaning (typically equipment is sterile if it can be proven that every spot of the machine has reached a temperature of 250°F (121°C) which has been maintained for at least 20 minutes).
There are many obvious advantages of incorporating clean-in-place technology to your system. It optimizes time (shortened cleaning cycles, no labor to disassemble parts or manually clean), improves the hygiene of the operation (which in turn improves the product quality), and provides an environmental benefit of more effectively using water, detergent and other resources needed in the cleaning process. In summary, CIP improves the overall efficiency of the process.
Criteria for Designing a Cleaning System
While there are certain aspects of CIP that are universal, there are also different options available based on equipment type and other elements that need to be considered. Filter/Dryers are different from glass-lined vessels, and therefore the cleaning system they employ will need to be different as well.
Some of the factors that come into consideration when designing a CIP system include:
- Complexity of equipment – geometry, configuration, material of construction, piping orientation, nozzle and discharge valve locations, etc.
- Characteristics of residuals – nature of contamination, chemical state of residue (solid/liquid), product’s compatibility with prospective cleaning agents, solubility, static properties, toxicity.
- Selection of a detergent/cleaning agent – avoidance of potential chemical reaction with product residue, solution type (acidic or alkaline, anionic, non-ionic, cationic, or amphoteric), concentration, contact time, temperature, etc.
- Cleaning equipment hardware – spray balls, spray rings, orientation hardware (stationery or rotating), quantity, installation location, hydrodynamic forces, high/low pressure options.
- Cleaning method – once through CIP, recirculating CIP, boil-up method (reflux), additional rinsing cycle.
Validating a Cleaning Procedure for the FDA
In many industries, the FDA sets requirements for cleaning process equipment. These good manufacturing practices are regulations put in place to ensure equipment is maintained in a clean and orderly fashion. Their main concerns are to avoid contamination of the product and cross-contamination (by improper cleaning, bad maintenance) both of which can lead to product call-backs.
If you work with a process that must meet FDA regulations, then you are probably aware of what the FDA requires from the user. The laundry list of items includes a written description of how the cleaning procedure is validated, including who is responsible for the approval of the validation, which acceptance criteria are defined, and when a re-validation will be required. Additionally, training must be proven, problem areas need to be identified, and a written validated cleaning procedure must be provided to show what will be done to ensure those problem areas will get cleaned in adherence to the required standards.
Riboflavin Testing
For the purpose of conducting a qualitative coverage test of the spray devices, a riboflavin test can be performed to measure the accuracy of the cleaning system (we’ll go more into detail next week about what this testing entails). The results of this procedure can be useful for the end-user in the development of cleaning validation protocols.
Cleaning System Options for Filter/Dryers
De Dietrich Process Systems offers many wash-in-place options for filter/dryers, ensuring complete cleaning of the interior and exterior of the vessel. We look at the location and quantity of components to make sure we adequately clean and deliver cleaning fluid to the unit. In addition, we look at the equipment design to reduce any areas of product hold up and improve drainability.
With each system, riboflavin testing is included as a comprehensive service to ensure the cleaning meets your requirements. Here are the various WIP/CIP choices for filter/dryers and what they can accomplish:
Spray Lance
 Designed to be removable from the vessel, spray lances introduce a standard rotating or fixed spray ball into the vessel. An O-ring seal is utilized to eliminate product within the nozzle projection. The spray lance can stay in the vessel during operation; however, it should be removed if the entire vessel capacity is to be used.
Designed to be removable from the vessel, spray lances introduce a standard rotating or fixed spray ball into the vessel. An O-ring seal is utilized to eliminate product within the nozzle projection. The spray lance can stay in the vessel during operation; however, it should be removed if the entire vessel capacity is to be used.
ARD Nozzles
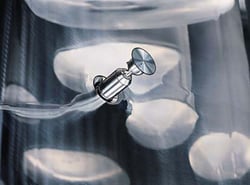 ARD (Aftertreatment Regeneration Device) nozzles are spray nozzle that are flush with the vessel interior during the process. Hydraulic pressure inserts the nozzle(s) during the cleaning procedure. Depending on the characteristics of the products used in the process, the nozzles can be constructed of metallic components or all PTFE. Standard sanitary connections can be used for solvent introduction.
ARD (Aftertreatment Regeneration Device) nozzles are spray nozzle that are flush with the vessel interior during the process. Hydraulic pressure inserts the nozzle(s) during the cleaning procedure. Depending on the characteristics of the products used in the process, the nozzles can be constructed of metallic components or all PTFE. Standard sanitary connections can be used for solvent introduction.
Mechanical Seal Spray Ring
 Mechanical seal spray rings are designed to clean the cavity created by the shaft penetration through the top head.
Mechanical seal spray rings are designed to clean the cavity created by the shaft penetration through the top head.
External Spray Ring for Top Head Nozzles
.jpg?width=250&height=334&name=External_spray_ring%20(1).jpg) Traditionally, the top head nozzles are known to be a difficult place to clean due to the nooks and crannies that are present in the nozzle design. External spray rings are built specifically for this purpose and feature a tangential entry spray jet via a wash solvent manifold that is connected to the top head nozzles with braided stainless steel flex hose.
Traditionally, the top head nozzles are known to be a difficult place to clean due to the nooks and crannies that are present in the nozzle design. External spray rings are built specifically for this purpose and feature a tangential entry spray jet via a wash solvent manifold that is connected to the top head nozzles with braided stainless steel flex hose.
Removable Spray Balls
 Fully removable from the top head, high volume spray balls are designed for full vessel coverage. This set up requires a single customer connection for each spray ball assembly (multiple spray balls are used in larger volumes to ensure coverage). For customers that want a more permanent fixture, welded spray balls are available as well.
Fully removable from the top head, high volume spray balls are designed for full vessel coverage. This set up requires a single customer connection for each spray ball assembly (multiple spray balls are used in larger volumes to ensure coverage). For customers that want a more permanent fixture, welded spray balls are available as well.
WIP/CIP/SIP systems are definitely worth looking into more if you don’t currently have one integrated in your process. By eliminating the need to manually clean, the “in-place” process saves you time while optimizing your equipment – that is efficiency at its best! DDPS can design and test systems to clean new and existing filter/dryers during refurbishment. Be on the lookout for next week’s post where we will talk about WIP systems for glass-lined vessels.
