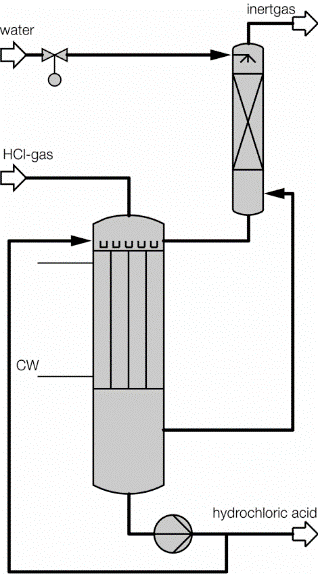
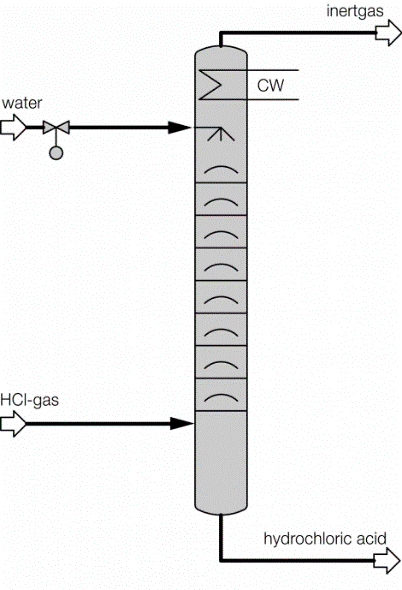
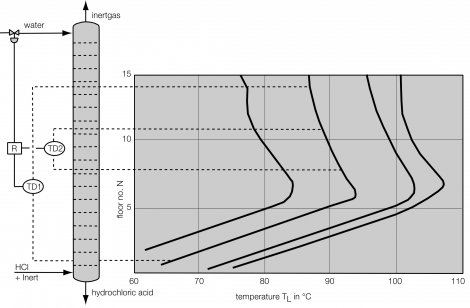
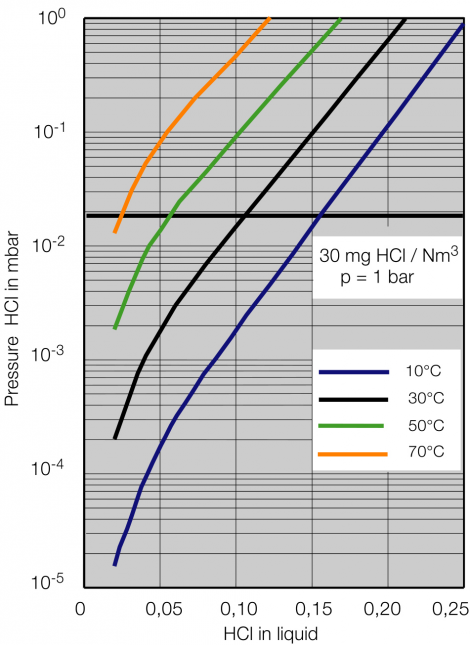
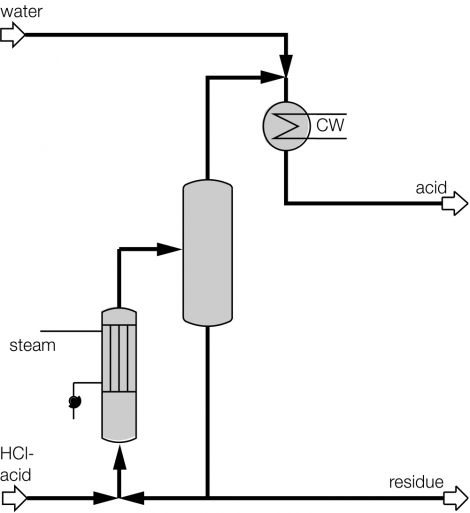
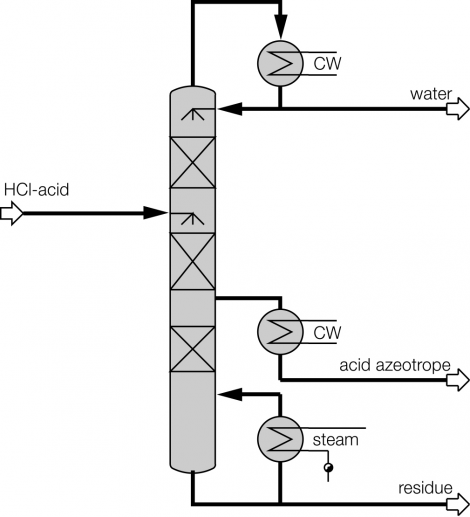
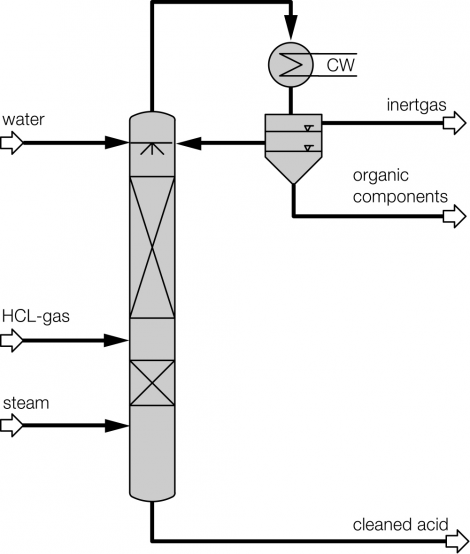
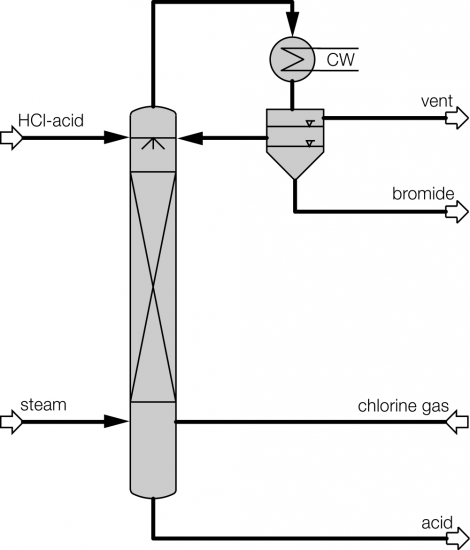
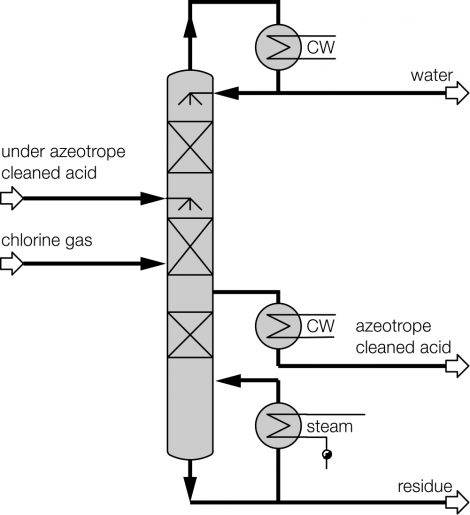
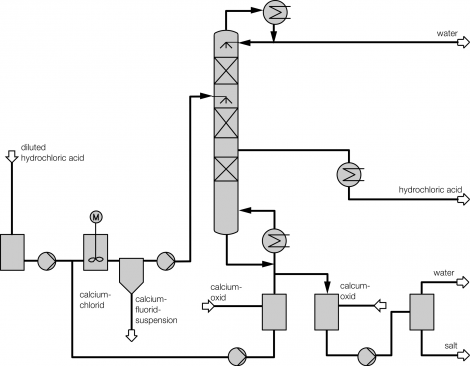
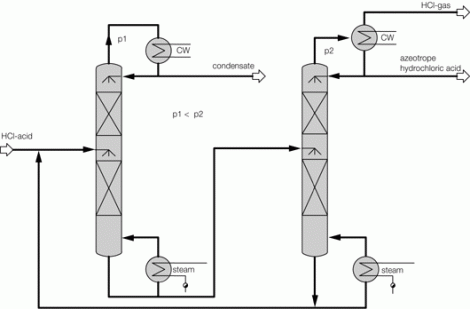
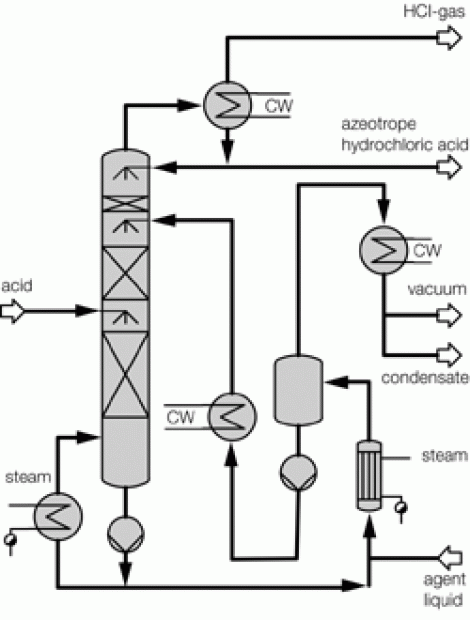
Process description combined technology for concentration
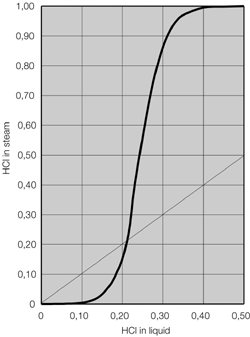
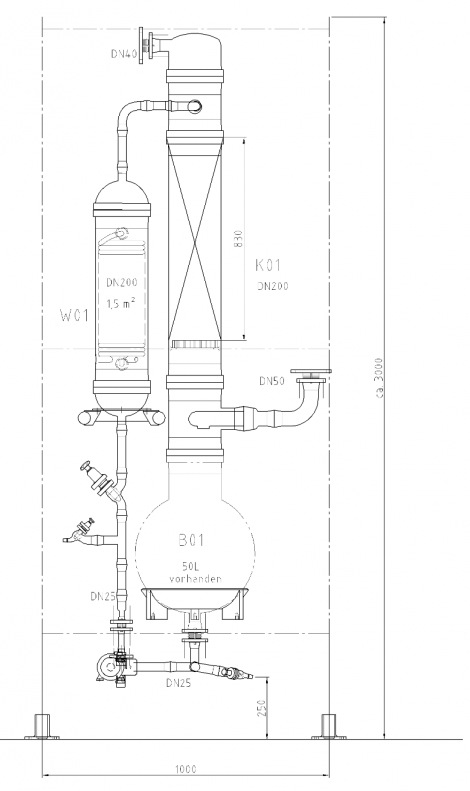
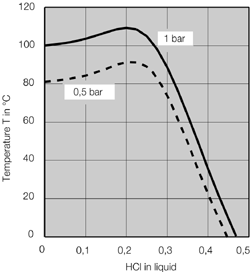
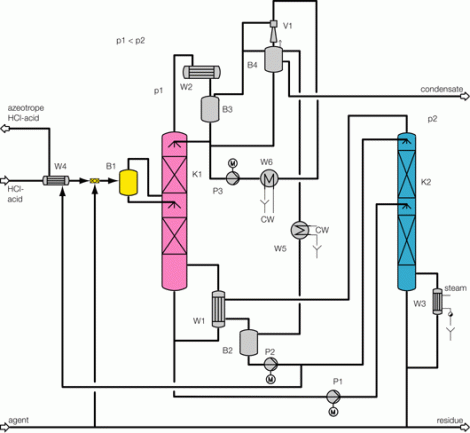
Illustration 4 above displays the flow chart of such a combined technology for the concentration of hydrochloric acid. The deployed raw acid is pre-heated in the feed pre-heater W4 against the concentrated hydrochloric acid; it is then mixed with the extractive agent, and then relaxed in the flash container B1. The resulting vapor is separated from the fluid in flash container B1, and feed into the middle of the vacuum column K1. The fluid from flash container B1 is also fed into the vacuum column K1 via an appropriate distributor. The mixture is rectified in vacuum column K1 so that water results in the column head and a mixture unfolds in the column bottom, whose composition almost matches to such found at a distillation borderline. This mixture is removed from the bottom of the vacuum column K1 via the pump P1, and fed into the pressure column K2.
The bottom mixture of the vacuum column is further rectified in the pressure column K2. A mixture now results as bottom product of pressure column K2 that matches to such found at the distillation borderline, and whose HCl concentration level is yet less than that of column K1 based on its system pressure. Thus, the bottom product of the pressure column K2 may therefore be recycled back to the column K1. For this, the bottom product of column K2 is relaxed in the flash container B1 together with the feed flow. As an extractive agent, the deployed ammonium chloride displays a high boiling point relative to the components of the feed acid; it then remains in the described fluid circulation. At the same time, employing ammonium chloride increases the relative volatility of HCl against water, so that the circulating volume of the described fluid circulation is minimized. In order to remove the contamination brought in by the feed flow, a partial flow is extracted from the bottom of the pressure column K2 and a specified amount of extractive agent is re-fed.
Concentrated hydrochloric acid of the desired concentration is produced at the head of the column K2. Vapor of the column K2 is condensed in the heat exchanger W1, which also serves as bottom heater of vacuum column K1. Achieving this heat integration minimizes energy deployment and thus operational costs of the plant. Hydrochloric acid of over-azeotrope concentration, which has condensed in heat exchanger W1, is collected in the distillation vessel B2, whereby a partial flow is re-fed to the top of pressure column K2 via hydrochloric acid pump P2. The main amount of the condensed hydrochloric acid is removed from distillation vessel B2 via the help of hydrochloric acid pump P2, and cooled down in feed pre-heater W4 against the feed flow, so that a cooled over-azeotrope hydrochloric acid flows as product from the concentration plant. Bottom heater W3, heated via saturated steam performs the heating of pressure column K2.
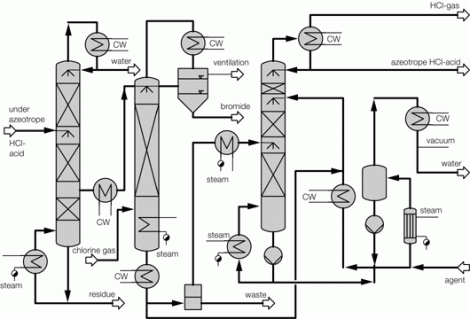
Generally, the above problem does not limit itself to sole absorption, extractive rectification, or cleaning of hydrochloric acid. Holistic solutions are frequently required, where an exhaust flow is cleaned while concentrated, cleaned hydrochloric acid is recycled.