Safely Charging Solids into Process Reactors
 While adding solids into a reactor is a common step in most reaction processes, it is often performed in ways that introduce serious safety risks. Even with seemingly stable materials, hazards can arise from dust or vapor clouds formed during manual charging.
While adding solids into a reactor is a common step in most reaction processes, it is often performed in ways that introduce serious safety risks. Even with seemingly stable materials, hazards can arise from dust or vapor clouds formed during manual charging.
Adding an inert gas to reduce the amount of oxygen in the vessel can help, but the benefits of this precautionary step are often voided once a manway is opened to introduce solids manually.
This post outlines key ways to reduce explosion risks when charging solids into process reactors.
How Explosive Conditions Develop in Reactors
Before you can prevent an explosion from occurring, it’s important to have a full understanding of what can cause one in a reactor. The most common causes of explosive conditions during solids addition include:
- Flammable solvent vapors
- Combustible dust clouds
- Hybrid dust / vapor cloud combinations
Since flammable vapors and explosive dust clouds are both likely to be present inside a reactor during solids introduction, it is important to consider not only what impact they could have independently, but also what effect a mixture of them might have on their individual ignition characteristics. For example, even if the concentration of vapor and dust is their individual explosive limits, the mixture of the two may fall within its upper and lower explosive limits.
Common Ignition Sources During Solids Charging
Once you’ve assessed the potential of an explosive atmosphere inside your reactor, the next step is identifying (and eliminating) any possible sources of ignition. In solids charging, the main sources for ignition include:
- Mechanical sparks – often caused by running the agitator during solid’s charging (to prevent large clumps from forming)
- High surface temperatures – produced by a potentially high vessel temperature in combination with frictional heat generated from an imbalanced mechanical seal.
- Electrostatic discharge – generated by the accumulation and discharge of static electricity, created from two material surfaces rubbing together (e.g. fluids flowing into vessels or solid particles rubbing against one another)
Processes often contain multiple ignition sources, making risk elimination extremely difficult.
Why Inerting Is Critical (But Not Always Enough)
While there are various methods for preventing explosions that can differ based on the uniqueness of your process and products, ultimately the most critical safety measure you can take is to inert the atmosphere in your vessel.
Atmospheric air contains approximately 21% oxygen. so By sufficiently reducing this level through inertion, it is possible to reach an oxygen concentration below which no ignition is possible. However, it’s important to note that inerting alone is not a complete safeguard. Even after a vessel has been inerted to a safe level, precautions must be taken to prevent reintroducing oxygen—particularly during solids charging. This is particularly important if manual or gravity feeding is used, i.e. manways are opened.
Dust Explosion Prevention Analysis: The Risk Pentagon
When considering the safest way to charge solids into a reactor, it’s essential to consider all five elements of the dust explosion pentagon. This framework helps assess the key conditions that contribute to explosion risk, and which can realistically be controlled or eliminated.
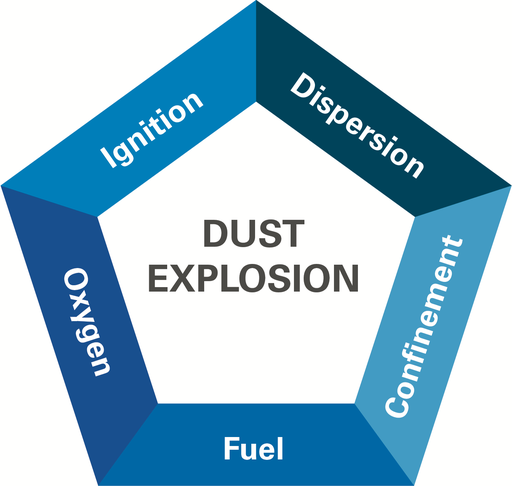
Since fuel and dispersion, in the form of flammable solvent vapors and accumulated dust clouds are likely to be present inside the vessel, there is no way to eliminate those portions of the pentagon (and the confined space of a vessel is a non-negotiable factor as well).
Ignition sources, such as heat or sparks due to mechanical failure or accidental discharge can never be prevented with 100% certainty, so an ignition source cannot be eliminated either. As a result, oxygen becomes the only factor in the pentagon that can be reliably controlled.
Removing or reducing oxygen through inerting remains the most effective way to prevent fires and explosions in solid charging operations.
A Safer Solution: Oxygen-Free Solids Charging
De Dietrich's Powder Handling Systems are is designed to do just this. Along with its static-dissipating materials and conductive design, it can provide an extremely safe method to introduce solids into a reactor by removing oxygen from the solids and replacing it with nitrogen prior to discharge into the reactor.
Looking for a Safer Solids Charging Strategy?
Are you looking for more information on the topic of safely charging powders? This post gives a very high-level overview of the problems that can arise when charging solids into a reactor. For a deeper dive into the subject, download our new eBook, Safely Charging Solids into Process Reactors.
You can also contact our powder handling team if you have additional questions about the Powder Pump and how our powder handling solutions might be a fit for your application.
