Case Study: Minimizing Bromine for Chlorine Electrolyzers
 If you have read our blog before, or are familiar with our product lines, you understand we specialize in glass-lined steel and borosilicate glass equipment. While these materials of construction are preferred and sometimes required for certain corrosive processes, there are also times, in the many industries we serve, that they are not the right fit for the application. This shouldn’t come as a surprise; part of being in a niche market means you are fulfilling a specific need and not a solution for everyone. There are instances, however, when a company’s expertise in a certain technology outweighs the other challenges of the project, and an alternative solution can be achieved.
If you have read our blog before, or are familiar with our product lines, you understand we specialize in glass-lined steel and borosilicate glass equipment. While these materials of construction are preferred and sometimes required for certain corrosive processes, there are also times, in the many industries we serve, that they are not the right fit for the application. This shouldn’t come as a surprise; part of being in a niche market means you are fulfilling a specific need and not a solution for everyone. There are instances, however, when a company’s expertise in a certain technology outweighs the other challenges of the project, and an alternative solution can be achieved.
A perfect example of such a project involves a debromination plant that De Dietrich Process Systems recently commissioned, made of non-DDPS material.
Debromination is a process that’s been in our wheelhouse for decades. To maximize the efficiency of these plants, the columns and their internals must be highly corrosion-resistant and specifically constructed for the process. Their task is to equally distribute large feed streams and to redistribute them along the column to avoid dead volumes and to maximize the mass transfer area. Our clients traditionally use QVF components and a process that involves either chlorine or hydrogen peroxide to reduce the bromide content in the brines down to as low as 10 ppm. While the above described process is pretty straightforward in design, the solution is not always as simple. Feeds are often contaminated with organic materials from the reactors and they must be removed by steam stripping before entering the stripping column; otherwise, they may react with bromine and consequently reduce the yield.
Project Background
A well-known chemical company launched a modern membrane-based chlorine electrolyzer to produce high quality chlorinated organic products. The 90t/h feed for this electrolyzer was a potassium chloride brine containing about 260ppm Br (0.026wt%). To fulfill the quality requirements for the final product, the bromine content had to be reduced to 30ppm. The total quantity of bromine generated from this feed per year is less than 190t/y; since this amount does not necessitate an investment in a bromine handling infrastructure, the bromides taken out of the feed were dumped with the waste waters. Environmental laws require that the waste waters of a debromination plant be neutral with respect to the redox-potential and to contain a minimal salt load.
To carry out these demands, the customer requested to use hydrogen peroxide to convert the bromine to harmless bromide. The debromination process that was developed to perform this chemical conversion could be carried out at temperatures less than 212°F, making it a “cold blow process”. Although highly corrosive, the lower temperature allowance made glass-lined steel or borosilicate glass an unnecessary build material for this plant.
When specialized materials of construction aren’t a necessity, they can become cost prohibitive to the project. Still, our vast experience with debromination projects made DDPS a strong contender among the list of manufacturers the customer was considering. Ultimately, DDPS was able to create a cost-effective solution focused on the final product requirements without using our niche materials of construction. To keep the expenses down, we offered a process system made of glass fiber reinforced plastic. This solution utilized PVDF structured packing in combination with the GFRP-constructed columns to effectively and economically improve the quality of the brine for the chlorine production.


Above: Insulated GFRP columns
Right: Insulated stripping and scrubbing columns
Solution Details
Based on the reduction of chlorine, the basic reaction for the debromination process is the same as the production of bromine:
2Br- + Cl2 → Br2 + 2CL-
Chlorine is available on site and the bromine is stripped off with air at less than 212°F. The bromine and the unconsumed accompanying chlorine are then chemically absorbed with sodium hydroxide in a scrubbing column:
3 Br2 + 6 NaOH → 5 NaBr + NaBrO3 + 3 H2O
Cl2 + NaOH → HCl + NaOCl
In a third step, the bromates and hypochlorides are reduced with hydrogen peroxide:
NaOCl + H2O2 → O2 + NaCl + H2O
NaBrO3 + 3 H2O2 → 3 O2 + NaBr + 3 H2O
The following flow chart illustrates the debromination process that was conceived:
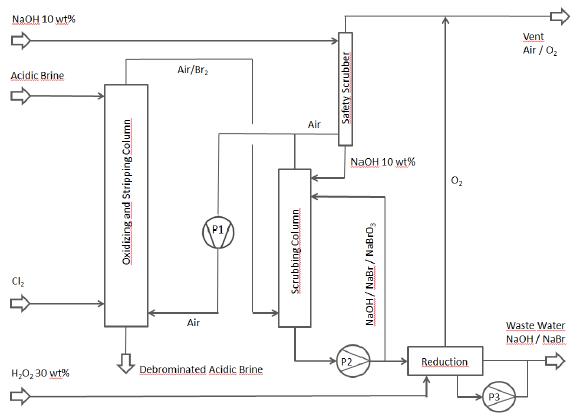


Gas circulation pumps Piping made of GFRP
This debromination plant case study demonstrates the importance of process know-how and how technical experience can sometimes supersede product offering. De Dietrich Process Systems has a unique combination of products and competences that cover a large array of applications. The ability to provide unmatched process expertise and to source different materials of construction when necessary enables us to find the best, most cost-effective solutions – even ones made of “non-DDPS” material. In combination with engineering, procurement, and construction capabilities, DDPS provides comprehensive systems for halide treatment, recovery/concentration/purification of mineral acids, waste water treatment, and more. For a free consultation, contact us online or fill out our Process Systems Questionnaire to give us more information about your process so we can discuss the needs of your business in greater detail.

